What Are the Five Properties Used to Classify a Substance as a Mineral?
Geologists have recently adamant that the minerals goethite and hematite exist in abundance on Mars, sure signs of the presence of water (run into Effigy 1 for a motion-picture show). None of those geologists take been to Mars, of course, simply the unmanned rovers Spirit and Opportunity accept. These rovers are equipped with three mass spectrometers, each of which is capable of determining the chemical composition of a solid with a high caste of accurateness. With such a precise chemical analysis in paw, geologists on Globe had no problem identifying the minerals.

A mineral is defined in role past a specific chemical composition. In theory, therefore, it is e'er easy to identify a mineral, if you can determine the chemical limerick with a mass spectrometer like the Mars rovers. In reality, however, even if you are looking at rocks on Earth, determining the exact chemical composition of a substance involves significant time preparing the sample and sophisticated laboratory equipment (and often significant money). Luckily, it is ordinarily unnecessary to go to such lengths, because there are much easier ways that crave little more than than a magnifying lens and a penknife.
Identifying minerals past physical properties
The most common minerals in Earth's crust can often exist identified in the field using bones concrete backdrop such as color, shape, and hardness. The context of a mineral is important, besides – some minerals can form nether the same conditions, so you are likely to find them in the same rock, while others form nether very unlike conditions and will never occur in the aforementioned rock. For this reason, context (the other surrounding minerals and type of rock) can oft exist used to rule out minerals that have similar colour, for example. Although there are many thousands of named minerals, but a dozen or so are common in Earth's crust. Testing a few physical backdrop therefore ways that you tin identify about 90% of what you are likely to encounter in the field.
Considering the physical properties of a mineral are determined by its chemical composition and internal atomic structure, they tin exist used diagnostically, the way a runny nose and sore throat can exist used to diagnose a cold. There are many physical properties of minerals that are testable with varying degrees of ease, including color, crystal form (or shape), hardness, luster (or smooth), density, and cleavage or fracture (how the mineral breaks). In addition, many minerals have unique properties, such every bit radioactivity, fluorescence under black light, or reaction to acid. In most cases, it is necessary to detect a few backdrop to place a mineral; to extend the medical analogy even farther, a runny nose is a symptom of a cold virus, allergies, or a sinus infection amidst other things, and then nosotros have to use other symptoms to diagnose the problem – a headache, fever, watery eyes, and and so on.
Comprehension Checkpoint
The context in which a mineral is found
Color
The most obvious property of a mineral, its color, is unfortunately besides the to the lowest degree diagnostic. In the aforementioned way that a headache is a symptom for a whole host of problems from the flu to a head injury, many minerals share the same colour. For example, several minerals are green in color – olivine, epidote, and actinolite, merely to proper name a few. On the other extreme, one mineral can have on several unlike colors if there are impurities in the chemical composition, such as quartz, which can exist clear, smoky, pink, purple, or yellow.
Function of the reason that the color of minerals is not uniquely diagnostic is that there are several components of the crystal compositions and structure that can produce color. The presence of some elements, such every bit iron, always results in a colored mineral, just iron can produce a broad variety of colors depending on its state of oxidation – black, reddish, or green, most commonly. Some minerals have colour-producing elements in their crystal structure, similar olivine (IrontwoSiOiv), while others incorporate them as impurities, like quartz (SiO2). All of this variability makes it difficult to solely use color to identify a mineral. However, in combination with other backdrop such equally crystal class, color tin can aid narrow the possibilities. As an example, hornblende, biotite, and muscovite are all very usually institute in rocks such as granite. Hornblende and biotite are both black, merely they can be easily distinguished by their crystal grade considering biotite occurs in sheets, while hornblende forms stout prisms (Figure 2). Muscovite and biotite both form in sheets, simply they are different colors – muscovite is colorless, in fact.

Comprehension Checkpoint
Color is ane of the best ways to identify a mineral.
Crystal class
The external shape of a mineral crystal (or its crystal grade) is determined largely by its internal diminutive construction, which means that this belongings can exist highly diagnostic. Specifically, the form of a crystal is defined by the angular relationships between crystal faces (call up Steno's Law of Interfacial Angles as discussed in our Minerals I module). Some minerals, similar halite (NaCl, or salt) and pyrite (FeS) have a cubic form (encounter Figure 3, left); others similar tourmaline (run across Effigy 3, middle) are prismatic. Some minerals, like azurite and malachite, which are both copper ores, don't form regular crystals, and are baggy (Effigy three).

Unfortunately, we don't always get to see the crystal form. We come across perfect crystals simply when they have had a gamble to grow into a crenel, such as in a geode. When crystals abound in the context of cooling magma, withal, they are competing for space with all of the other crystals that are trying to grow and they tend to fill in whatever space they tin. The shape of the crystal can vary quite a bit depending on the amount of space available, but the angle between the crystal faces volition always be the same.
Comprehension Checkpoint
Which is more helpful in identifying a mineral?
Hardness
The hardness of a mineral can be tested in several ways. Most commonly, minerals are compared to an object of known hardness using a scratch test – if a smash, for case, can scratch a crystal, than the nail is harder than that mineral. In the early 1800s, Friedrich Mohs, an Austrian mineralogist, adult a relative hardness scale based on the scratch test. He assigned integer numbers to each mineral, where 1 is the softest and 10 is the hardest. This scale is shown in Effigy 4.
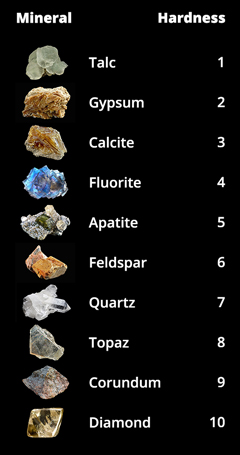
The calibration is non linear (corundum is really four times as hard as quartz), and other methods accept now provided more than rigorous measurements of hardness. Despite the lack of precision in the Mohs scale, information technology remains useful because it is unproblematic, like shooting fish in a barrel to remember, and easy to test. The steel of a pocketknife (a common tool for geologists to acquit in the field) falls almost right in the heart, so it is easy to distinguish the upper half from the lower half. For example, quartz and calcite can look exactly the same – both are colorless and translucent, and occur in a wide variety of rocks. But a elementary scratch test can tell them apart; calcite will be scratched by a pocketknife or rock hammer and quartz will not. Gypsum can besides wait a lot like calcite, but is and then soft that it can be scratched by a fingernail.
Variations in hardness make minerals useful for different purposes. The softness of calcite makes it a popular material for sculpture (marble is fabricated up entirely of calcite), whereas the hardness of diamond means that it is used as an abrasive to shine stone.
Comprehension Checkpoint
The hardness of a mineral can be adamant past attempting to scratch it with a knife.
Luster
The luster of a mineral is the way that it reflects light. This may seem similar a difficult stardom to make, but picture the difference between the way light reflects off a drinking glass window and the style information technology reflects off of a shiny chrome auto bumper. A mineral that reflects lite the way glass does has a vitreous (or glassy) luster; a mineral that reflects light like chrome has a metal luster. There are a variety of boosted possibilities for luster, including pearly, waxy, and resinous (see pictures in Figure five). Minerals that are as brilliantly reflective every bit diamond have an adamantine luster. With a niggling practice, luster is as hands recognized every bit color and tin can be quite distinctive, particularly for minerals that occur in multiple colors like quartz.

Density
The density of minerals varies widely from about 1.01 g/cmiii to near 17.5 g/cm3. The density of h2o is 1 yard/cm3, pure fe has a density of 7.6 g/cmiii, pure gold, 17.65 one thousand/cm3. Minerals, therefore, occupy the range of densities between water and pure gold. Measuring the density of a specific mineral requires fourth dimension-consuming techniques, and most geologists accept developed a more intuitive sense for what is "normal" density, what is unusually heavy for its size, and what is unusually lite. By "hefting" a rock, experienced geologists tin can usually guess if the rock is made upwardly of minerals that contain iron or lead, for example, because information technology feels heavier than an average rock of the same size (see our Density module for more information).
Cleavage and fracture
Most minerals contain inherent weaknesses within their diminutive structures, a plane along which the bond strength is lower than the surrounding bonds. When hit with a hammer or otherwise cleaved, a mineral will tend to break forth that plane of pre-existing weakness. This type of breakage is called cleavage, and the quality of the cleavage varies with the strength of the bonds. Biotite, for instance, has layers of extremely weak hydrogen bonds that break very easily, thus biotite breaks along apartment planes and is considered to have perfect cleavage (see Figure 6). Other minerals cleave along planar surfaces of varying roughness – these are considered to take good to poor cleavage.

Some minerals don't accept any planes of weakness in their atomic construction. These minerals don't have whatsoever cleavage, and instead they fracture. Quartz fractures in a distinctive fashion, called conchoidal, which produces a concave surface with a serial of arcuate ribs like to the way that glass fractures (see Figure vi). For quartz, in fact, this lack of cleavage is a distinguishing property.
Comprehension Checkpoint
A mineral with perfect cleavage
Mineral classification systems
Concrete properties provided the main ground for nomenclature of minerals from the Middle Ages through the mid-1800s. Minerals were grouped according to characteristics such as hardness, so that diamond and corundum would exist in the same class of minerals. As the ability to make up one's mind the chemic composition of minerals adult, and then did a new nomenclature system. Many scientists contributed to the discovery of mineral chemical formulas, just James Dwight Dana, a mineralogist at Yale University from 1850 to 1892 (encounter Biography link in the Resources section), developed a classification system for minerals based on chemic composition that has survived to the nowadays 24-hour interval. He grouped minerals according to their anions, such as oxides (compounds with O2-), silicates (compounds with (SiOiv)4-), and sulfates (compounds with (SO4)ii-). A chemical classification organization meant that minerals that were grouped together theoretically also tended to appear with each other in rocks since they tended to develop under like geochemical conditions.
Concrete properties still provide the main means for identification of minerals, however, though they are no longer used to group minerals (from the case to a higher place, corundum is an oxide while diamond is a pure element, so by Dana'south system, they are in separate groups). A composition-based grouping highlights some common mineral associations that allow geologists to make educated guesses about which minerals are present in a rock, even with just a quick glance. By far, the nigh common minerals are the silicates, which make upward 90% of Earth's crust. Of the many hundreds of named silicate minerals, just about eight are common, ane of which is quartz. The uncommon minerals are critical, however, as they include economically important ones such as galena, which is the principal ore for lead, and apatite, a phosphate mined for phosphoric acrid that is added to fertilizers. The discovery of new ore deposits depends on the ability of geologists to identify what they see in the field and recognize unusual mineral occurrences that should exist explored in more detail in the laboratory. A mitt lens, a pocketknife, and a lot of practice still provide the easiest and cheapest methods of identifying minerals.
Summary
Minerals are classified on the basis of their chemic limerick, which is expressed in their concrete properties. This module, the 2nd in a series on minerals, describes the physical properties that are unremarkably used to identify minerals. These include color, crystal form, hardness, density, luster, and cleavage.
Primal Concepts
-
Properties that help geologists identify a mineral in a rock are: color, hardness, luster, crystal forms, density, and cleavage.
-
Crystal form, cleavage, and hardness are determined primarily by the crystal construction at the atomic level.
-
Color and density are determined primarily past the chemical composition.
-
Minerals are classified on the footing of their chemic composition.
Source: https://www.visionlearning.com/en/library/Earth-Science/6/Properties-of-Minerals/130
0 Response to "What Are the Five Properties Used to Classify a Substance as a Mineral?"
Postar um comentário